GenesWell BCT Reclassifies Subset of Oncotype DX Low-Risk Patients
The retrospective study included 759 patients with early-stage hormone receptor-positive, HER2-negative (HR+/HER2-) breast cancer, treated across five institutions in Korea: Gangnam Severance Hospital, Samsung Medical Center, Asan Medical Center, National Cancer Center, and Korea University Guro Hospital. This study builds upon prior research assessing risk classification concordance in 2019 and follows findings presented at the 2023 ASCO Annual Meeting.
Published in Frontiers in Oncology, the study assessed recurrence-free survival (RFS) using both the GenesWell BCT score and the Oncotype DX recurrence score.
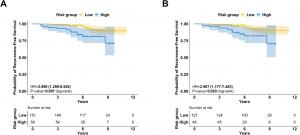
In the overall patient cohort, GenesWell BCT stratified patients into low- and high-risk groups with a 7-year RFS of 95.0% and 88.4%, respectively, compared to 93.7% and 88.1% with Oncotype DX. The hazard ratio (HR) between high- and low-risk groups was 2.469 (95% CI: 1.446–4.214) for GenesWell BCT and 2.093 (95% CI: 1.138–3.850) for Oncotype DX.
Among patients classified as low-risk (RS ≤25) by Oncotype DX, the GenesWell BCT score reclassified a subset into a high-risk group, where a statistically significant difference in RFS was observed (HR=2.477).
The study further assessed 209 women aged 50 years or younger with intermediate Oncotype DX recurrence scores (RS 16–25). In this subgroup, the GenesWell BCT score stratified recurrence risk with a statistically significant difference in RFS between low- and high-risk groups (P=0.007). The difference remained significant among patients who did not receive chemotherapy (P=0.020).
Dr. Sung Gwe Ahn, lead author and breast surgeon at Gangnam Severance Hospital, stated:
"This study, based on more than seven years of follow-up, suggests that patients classified as low-risk by Oncotype DX may still have a considerable risk of recurrence."
Prof. Sae Byul Lee of Asan Medical Center commented:
"The findings may help inform chemotherapy decision-making, particularly for younger breast cancer patients."
GenesWell BCT has received regulatory approval from the Ministry of Food and Drug Safety (MFDS) in Korea and is listed under the national health insurance code.A post-market surveillance (PMS) study enrolling 600 patients with 10 years of follow-up has been conducted to evaluate long-term outcomes.
Dr. Sang Uk Woo of Korea University Guro Hospital noted:
"GenesWell BCT has been evaluated in Korean patients, with long-term follow-up studies supporting assessment of clinical outcomes."
Gencurix continues to provide GenesWell BCT as a diagnostic service in several Asian countries and is pursuing additional regulatory submissions and insurance reimbursement approvals in international markets.
Gencurix
Gencurix
homepage_qna@gencurix.com
Distribution channels: Business & Economy, Companies, Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release